Categories
Tags
-
#Sterile API facility design consultants in India
#Cosmetics facility design consultants in India
#HVAC design for pharma manufacturing
#cGMP facility design consultancy
#Pharma engineering services for biotech and injectables
#facility design
#OSD (Oral Solid Dosage) plant design consultancy
#HVAC & MEP Design for Pharma
#Top pharma facility design consultants worldwide
#Clean Room Deisgn Consultants India
#Pharma plant design & validation services
Archives
Clean Room Design Consultants India: Engineering Controlled
-
In India’s rapidly expanding pharmaceutical, biotech, medical device, electronics, and semiconductor industries, cleanrooms are no longer optional infrastructure — they are regulatory necessities.
But here’s the reality:
Clean Room Design Consultants in India deliver precision-engineered environments tailored for pharmaceutical, biotech, and healthcare industries. From airflow planning and HVAC integration to GMP compliance and validation support, they ensure contamination control and seamless regulatory approvals. With strategic layout planning and advanced engineering solutions, these experts create audit-ready facilities that meet global quality standards.
A cleanroom is not just a sealed room with HEPA filters. It is a scientifically engineered contamination control system. And that’s where professional Clean Room Design Consultants in India play a decisive role.If you’re planning a GMP facility, sterile injectable plant, OSD unit, biotech lab, or medical device manufacturing site — this guide explains how expert cleanroom consultants turn compliance requirements into operational excellence.
- Why Clean Room Design in India Requires Specialized Expertise
India is one of the largest pharmaceutical manufacturing hubs globally. Facilities must comply with:
- WHO-GMP
- USFDA
- EU GMP
- CDSCO
- ISO 14644
- Schedule M (Revised)
A minor airflow imbalance or poorly designed pressure cascade can result in major regulatory observations.
Professional cleanroom design consultants ensure:
Contamination control
Energy-efficient HVAC
Proper zoning & segregation
Regulatory-ready documentation
Future scalability- What Clean Room Design Consultants in India Actually Do
- Concept Planning & Feasibility
Before civil work begins, consultants define:
- Cleanroom classification (ISO 5, ISO 7, ISO 8)
- Industry application (pharma, biotech, electronics, medical device)
- Product sensitivity
- Required pressure cascade strategy
- HVAC capacity & redundancy
Strategic planning at this stage prevents costly design corrections later.
- Cleanroom Layout & Zoning Strategy
4
Cleanroom consultants focus heavily on:
- Logical material & personnel flow
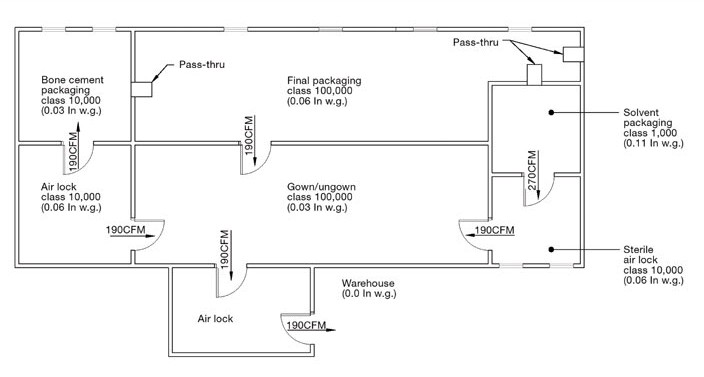
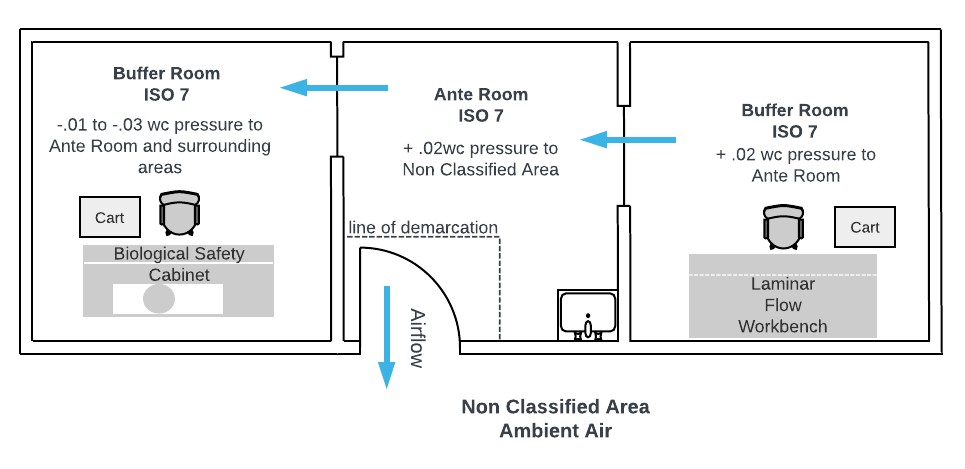
- Airlock placement
- Gowning room configuration
- Pass boxes & dynamic pass-through systems
- Segregated production zones
- Cross-contamination prevention
An efficient layout improves productivity and audit readiness.
- HVAC & Airflow Engineering
HVAC is the backbone of any cleanroom.
Expert consultants design:
- HEPA/ULPA filtration systems
- Unidirectional airflow (LAF/UDAF)
- Pressure differential cascade (10–15 Pa typical)
- Temperature & humidity control
- Energy-efficient air handling units
- Return air strategies
Improper HVAC design is the most common reason for compliance failures.
- Modular Cleanroom Construction
Modern Indian facilities increasingly prefer modular cleanroom systems because they offer:
- Faster installation
- Smooth flush panels
- Easy maintenance
- Scalability
- Reduced contamination risk
Consultants select panel materials, coving, flooring (epoxy/PU), and ceiling grids according to application risk.
- Validation & Compliance Support
Top cleanroom design consultants in India also assist with:
- Design Qualification (DQ)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Airflow visualization (smoke studies)
- HEPA integrity testing
- Particle count validation
Design and validation must work together for successful audits.
- Industries Served by Clean Room Consultants in India
- Pharmaceutical Manufacturing
Sterile and non-sterile production suites
- Biotechnology & Vaccine Plants
Aseptic and biosafety facilities
- Medical Device Manufacturing
ISO-classified assembly rooms
- Electronics & Semiconductor
Dust-free micro-component manufacturing
- Research Laboratories
Controlled R&D environments
- Common Cleanroom Design Mistakes to Avoid
Incorrect pressure cascade planning
Poor air change rate calculation
Inadequate return air design
Overdesigning HVAC (leading to high energy cost)
Ignoring maintenance accessibility
Improper airlock positioningA consultant’s role is to balance compliance with cost-efficiency.
- Key Features of a Future-Ready Cleanroom in India
Energy-efficient HVAC with VFD controls
Smart Building Management Systems (BMS)
Real-time environmental monitoring
Modular, scalable panel systems
Integrated fire & safety systems
Sustainable design with energy recoveryWith increasing energy costs in India, optimized cleanroom HVAC design is becoming a competitive advantage.
- How to Choose the Right Clean Room Design Consultant in India
Before finalizing a consultant, evaluate:
- Experience with Regulatory Audits
Have they supported USFDA or EU GMP inspections?
- HVAC Engineering Capability
Do they design in-house or outsource critical calculations?
- Industry Specialization
Pharma cleanrooms differ from electronics cleanrooms.
- End-to-End Support
Concept → Design → Execution Support → Validation
- Knowledge of Indian Regulations
Understanding Schedule M updates is critical.
- Why Professional Cleanroom Design Consultancy Saves Cost Long-Term
While consultancy fees may seem like an additional expense, they prevent:
- Rework and reconstruction
- HVAC oversizing
- Regulatory observations
- Production downtime
- Poor energy efficiency
A well-designed cleanroom operates smoothly for decades.
- The Future of Clean Room Design in India (2026 & Beyond)
India’s cleanroom industry is evolving toward:
- Smart cleanrooms with IoT integration
- Sustainable HVAC systems
- Modular plug-and-play production suites
- AI-based environmental monitoring
- Rapid deployment pharma facilities
The demand for specialized clean room design consultants in India is growing as compliance expectations become stricter globally.
- Final Thoughts
Clean Room Design Consultants in India are not just engineers — they are contamination control strategists, compliance advisors, and long-term infrastructure planners.
Whether you’re planning:
- A pharmaceutical cleanroom
- A sterile injectable unit
- A medical device facility
- An electronics manufacturing space
- Or a GMP-compliant expansion
Choosing the right cleanroom design consultant ensures regulatory approval, operational efficiency, and long-term sustainability.
